AZP2006 (Ezeprogind®)
AZP2006 is a first-in-class, orally available small molecule that stabilizes the progranulin–prosaposin (PGRN–PSAP) axis to restore lysosomal function, reduce Tau pathology, and attenuate neuroinflammation. Developed initially for Progressive Supranuclear Palsy (PSP), it has potential across a spectrum of neurodegenerative diseases including Alzheimer’s and Parkinson’s. Its multimodal profile sets it apart from the largely single-target approaches of the past 25 years.
Why PSP first
PSP is a rare primary tauopathy characterized by rapid progression, severe disability, and absence of disease-modifying therapies. Average survival from onset is 5–7 years. The clear clinical trajectory, validated rating scales, and urgent unmet need make PSP the optimal first indication to demonstrate efficacy of AZP2006.
- Key drivers: Tau aggregation, lysosomal failure, neuroinflammation
- Validated endpoints: PSP Rating Scale (PSPRS-28 and PSPRS-10)
- Orphan Drug Designation: Europe and USA
By targeting convergent pathogenic mechanisms, AZP2006 has the potential not only to slow PSP progression but also to be translated into larger indications such as Alzheimer’s and Parkinson’s. Both disorders share lysosomal impairment, Tau or α-synuclein pathology, and inflammatory responses as central drivers of neuronal death.
Lysosomal dysfunction is a hallmark of neurodegenerative diseases.
When lysosomes fail, toxic proteins and lipids accumulate, driving cellular stress, inflammation, and neuronal death. Genetic evidence links lysosomal defects to Alzheimer’s, Parkinson’s, frontotemporal dementia, and PSP. Restoring lysosomal function therefore represents a true disease-modifying approach across multiple disorders. Mutattions in the endo-lysosomal system have been linked to Alzheimer's disease (AD), Parkinson's disease (PD) and frontotemporal dementia (FTD)
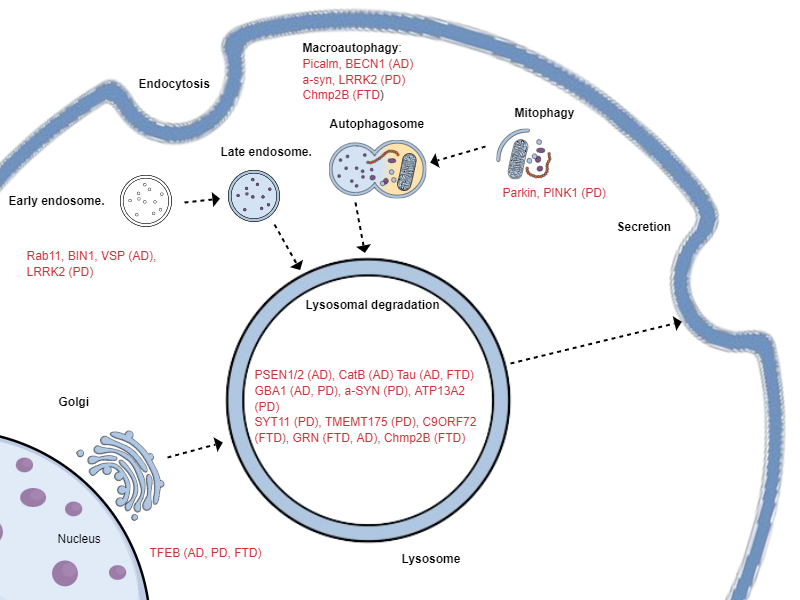
PGRN–PSAP Synergy in Lysosomal Health
Progranulin (PGRN) and Prosaposin (PSAP) form a cooperative axis essential for lysosomal function. Each protein promotes the trafficking of the other to the lysosome, ensuring efficient delivery and activation of degradative enzymes. This synergy preserves proteolytic capacity, maintains lipid homeostasis, and prevents the accumulation of toxic protein aggregates that drive neurodegeneration
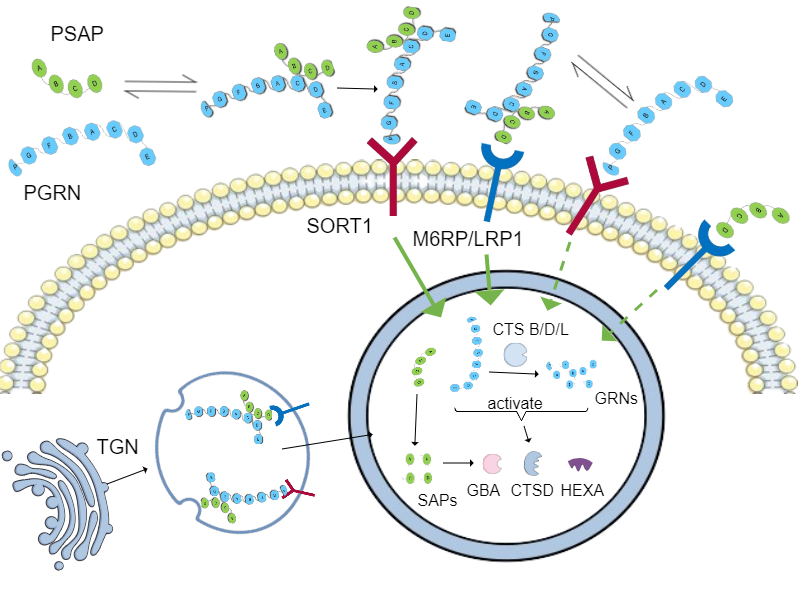
Mechanism of action
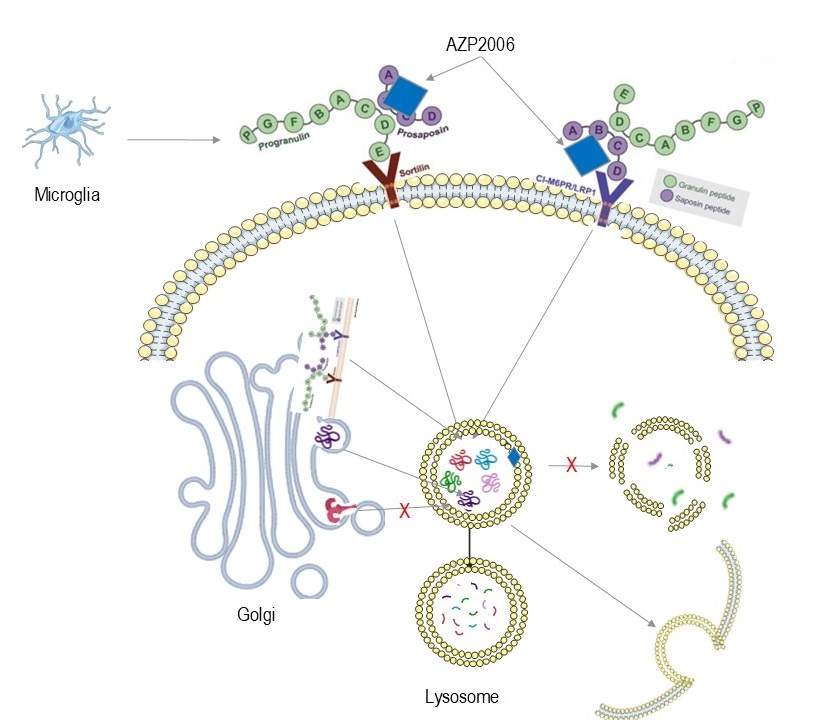
AZP2006 acts as a molecular chaperone that stabilizes the full-length progranulin–prosaposin (PGRN–PSAP) complex and enhances its trafficking via Sortilin, M6PR, and LRP1 receptors. This promotes delivery of intact PGRN and PSAP to lysosomes, reinforcing membrane integrity and boosting proteolytic capacity.
- Restores lysosomal homeostasis
- Reduces hyperphosphorylated Tau and misfolded protein accumulation (Abeta, Alpha-Syn, ...)
- Attenuates neuroinflammation and oxidative stress
- Supports neurite outgrowth and synaptic connectivity
This lysosome-centric MoA addresses core pathologies shared across PSP, Alzheimer’s, and Parkinson’s.
Preclinical evidence
- Tauopathy models: ↓ hyperphosphorylated Tau, ↑ neuronal survival, improved motor and cognitive performance
- Lysosome & inflammation: ↑ lysosomal activity and integrity, ↓ microglial activation, ↓ oxidative stress
- Connectivity: nanomolar modulation of PGRN and PSAP release, promotion of neurite outgrowth in vitro, enhanced synaptic connectivity
Clinical development
Phase 1 (SAD/MAD)
- Healthy volunteers
- Well tolerated; no serious adverse events
- Pharmacokinetics characterized; no hERG or cardiac signal
Phase 2a (PSP) + OLE
- Randomized, double-blind, placebo-controlled (36 PSP patients)
- Encouraging signals of clinical stabilization
- Open-Label Extension: supportive trends, especially with early initiation
Next step: larger, longer PSP trial to evaluate disease-modifying potential, as part of international platform initiatives.
Beyond PSP
AZP2006’s mechanism extends beyond PSP. In Alzheimer’s, lysosomal dysfunction and Tau aggregation drive neuronal death; AZP2006’s dual effect on lysosomes and Tau offers a differentiated strategy compared to amyloid-only approaches. In Parkinson’s, lysosomal impairment and α-synuclein accumulation are central; AZP2006 may reduce α-synuclein pathology by enhancing lysosomal clearance and limiting inflammatory cascades. This broad mechanistic convergence positions Ezeprogind® as a potential platform therapy across tauopathies and synucleinopathies.
Publications & resources
- AZP2006, a new promising treatment for Alzheimer’s and related diseases (2021): https://pmc.ncbi.nlm.nih.gov/articles/PMC8376949/
- AZP2006 (Ezeprogind®): a Promising New Drug Candidate in the Battle Against Neurodegenerative Diseases (2025): https://pubmed.ncbi.nlm.nih.gov/40150858/
- First-In-Human Safety, Tolerability, and Pharmacokinetics of Single and Multiple Doses of AZP2006, A Synthetic Compound for the Treatment of Alzheimer's Disease and Related Diseases (2024): https://pubmed.ncbi.nlm.nih.gov/38427472/
- AZP2006 in Progressive Supranuclear Palsy: Outcomes from a Phase 2a Multicenter, Randomized Trial, and Open-Label Extension on Safety, Biomarkers, and Disease Progression (2025): https://movementdisorders.onlinelibrary.wiley.com/doi/10.1002/mds.70049